Generating Foot-and-mouth disease resistant pigs
through gene-editing
----Novel solution to disease controlling
Project in a nutshell

From Genetic Literacy Project
Foot-and-mouth-disease (FMD) is one of the most important infectious diseases of domestic animals. It is highly contagious and has the ability to infect all cloven-hoofed animals including domestic livestock (e.g. sheep, cattle, pig) and various wildlife species. FMD outbreaks have caused severe economic consequences, for example, around £8 billion was spent on controlling the disease in the UK in 2001 (Perry. 2007).
FMD is caused by foot-and-mouth-disease virus (FMDV), a positive strand RNA virus that belongs to the Aphthovirus genus of the Picornaviridae family. Up to now, seven serotypes of FMDV (type O, A, C, SAT1, SAT2, SAT3 and Asia 1) have been identified, along with numerous subtypes that fall into these categories. The complex nature of FMDV make disease control and prevention challenging: there isn’t any vaccination available that could provide long-lasting protection against all serotypes of FMDV.
With the development of genomic technology, gene-editing may provide an alternative solution to FMD prevention. Our lab aims to identify and knock-out pro-viral genes that affects FMDV replication, ultimately generate FMD-resistant pigs using CRISPR/cas9 gene editing.
Genome scale siRNA knock-down screen was performed previously by the Haas group in order to identify novel genes that may be associated with FMDV replication. 53 human genes were identified and are proposed to either enhance or inhibit the viral replication, however, further confirmation in porcine cells is needed.
In this project, 4 genes that showed pro-viral activities in the screening were selected to generate porcine cell lines with genetic knock-out using CRISPR/Cas 9 gene-editing. The ultimate goal of the project is to generate FMDV resistant pigs.
The 4 genes are:
Plasminogen activator tissue type (PLAT)
Mannose receptor C type 2 (MRC2)
Hepatitis A virus cellular receptor 2 (HAVCR2)
Caveolin 3(CAV3)
By the end of the project, cell lines with PLAT knock-out were successfully generated and were tested. Although PLAT appear to have a pro-viral effect on FMDV replication, further confirmation is required.
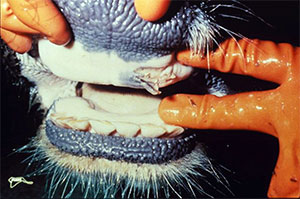
Vesicle developed in mouth of a cow.
From United States Department of Agriculture
The host range of FMDV covers most members of Arteriodactyla. Domestic farm livestock including cattle sheep and pigs are particularly vulnerable to FMD infection.
The water buffaloes, commonly involved in agricultural activities in Asia, may also be infected and can pass the disease to other livestock. Some wild animals (e.g. African buffaloes and impalas) are carriers of FMDV and may contribute to the natural epidemiology of FMD when they come in contact with susceptible livestock.
Clinal symptoms of FMD typically include fever, reduced appetite, development of severe vesicular disease and abortion in adult animal. Death is often associated with young animals.
FMDV can be transmitted by having direct contact with infected animals or indirect contact with contaminated products. The most common route for transmission is through the exchange of droplet nuclei (aerosol) between animals. Aerosol excreted by infected animals can be inhaled by recipients and be deposited in their respiratory tract. Indirect contact with contaminated animal products or tools is another important mean of transmission pathway. Virus may be contained in milk or meat products that are frozen and transported, therefore facilitate the spread of FMD to distant area. For this reason, the export and trading of animal products from FMDV endemic country are banned by law, which cause great economic loss to the country (OIE, FAO. 2012).
Different options are available to countries that suffer from FMD outbreak, depending on their trading status:
For FMD-endemic countries, vaccination and movement restriction are the main control methods. However, the current serotype-specific vaccine will not provide protection against other serotypes and subtypes. Furthermore, the lack of live-attenuated vaccine and the rapid mutation rates in virus make the vaccine protection short-lasting and can only last for approximately 6 months. This means that vaccination to animals is required twice a year, and that the cost for farming significantly increases.
For previously FMD-free countries such as countries in Europe, they also have the option to use vaccines at a price, meaning that they would have to give up their trading status for animal products. Therefore, culling of infected animals is most often employed, which causes catastrophic economic losses (OIE, FAO., 2012).
Our approach
image from synthego
Our laboratory aims to identify genes that affect FMDV replication, and by knocking-out pro-viral genes using CRISPR/Cas9 gene editing technique, it may be possible to generate FMD-resistant pigs. In previous work, 55 human genes have been identified and proposed to be pro-viral. In this project, four candidate genes (PLAT, MRC2, HAVCR2, and CAV3) were selected to generate porcine cell lines with genetic knock-out. Four experiments were carried out.
In the first experiment, as part of gene-editing process, guide RNA (gRNA) designed for the four target genes were first inserted into plasmids. gRNA helps to anchor the position of the DNA for editing. A total of 12 gRNA plasmids were produced, with three for each of the target genes.
In the second experiment, plasmids inserted with the gRNA specifically designed for PLAT gene were used to transfect a wild-type (WT) (or unchanged) porcine cells PK15. A total of 11 mutated porcine cell lines were identified with different forms of mutations: homozygous, mosaic and biallelic mutation.
In the third experiment, the effect of PLAT mutation on FMDV replication were studied using replicon assay. WT porcine cells were used as control. With the 4 mutant cell lines tested, different degrees of reduction in viral replication was seen. Compared with WT, PLAT-44-1, PLAT-44-3 and PLAT-44-4 had a reduction in viral replication by 2, 4 and 3 folds respectively and the mutation in PLAT-44-3 appeared to be most effective in resisting viral infection.
In the fourth experiment, replicon assay was performed on PK15 cells transfected with human PLAT siRNA, known to silence (or knock-down) the human PLAT gene. Results of this study did not show that the human siRNA had a silencing effect on the porcine cells. The experiment should be repeated using porcine PLAT specific siRNA.
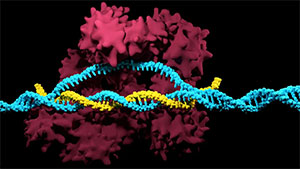
CRISPR/Ca9 gene-editingimage from Synthego
CRISPR/cas9 editing has produced promising outcomes in generating virus-resistant pigs (e.g. PRRSV, TGEV and ASFV) (Whitworth et al., 2016; Whitworth et al., 2019; Lillico et al., 2016), showing great potential in generating FMDV-resistant livestock.
Details on how gene-editing on livestock works can be found on:
https://academic.oup.com/af/article/9/3/6/5522878
Details on how CRISPR/Cas9 works can be found on:
https://www.synthego.com/learn/crispr